Tools
LyoPRONTO: An Open Source Lyophilization Process Optimization Tool
This work presents a new user-friendly lyophilization simulation and process optimization tool, freely available under the name LyoPRONTO. It is a new lyophilization simulation and process optimization tool. It includes freezing, primary drying modeling and optimization modules as well as the design space generator (Figure 1(b)). It can be used to model the lyophilization process and create more efficient cycles. Moreover, the tool is capable of determining the vial heat transfer parameters and product resistance characteristics, thus reducing the number of experiments.
The 0D lumped capacitance modeling approach is used in a freezing calculator to predict the product temperature variation with time and results in reasonably good agreement with experimental measurements. The primary drying calculator is based on 1D heat and mass transfer analysis (Figure 1(a)) in a vial and deviation from the experimental data is within 3% (Figure 1(d)).
The optimization tool enables acceleration of the primary drying cycle by 62% for 5% Mannitol (Figure 1(c)) and by 50% for sucrose solutions in comparison to traditional cycles. Thus, coupling with controllers and accurate sensors will allow creating self-driving lyophilizers.
For more information and to launch LyoPRONTO, visit our website
Training tools:
Fundamentals of Freeze Drying I: Introduction and Process Overview (Advantages and limitations of freeze drying, Product quality attributes, Process overview: freezing, primary drying, secondary drying)
Fundamentals of Freeze Drying II: The Freezing Process (Supercooling and ice nucleation, Characterization of freezing behavior, Establishment of upper product temperature limit during primary drying)
Fundamentals of Freeze Drying III: Primary and Secondary Drying (Primary Drying: heat transfer considerations, measurement of the vial heat transfer coefficient, mass transfer considerations, measurement of the resistance of the dried product layer and Secondary Drying: critical process variables during secondary drying, how dry is "dry enough")
Fundamentals of Freeze Drying IV: Process Monitoring
Fundamentals of Freeze Drying V: Formulation Considerations
The Fundamentals of Freeze Drying modules are presented by Dr. Steve Nail, Senior Research Scientist at Baxter Biopharma Solutions
 |
The Fundamentals of Freeze Drying modules are presented by Dr. Steve Nail, Adjunct Professor, Purdue University, retired Senior Research Scientist at Baxter Biopharma Solutions Dr. Steven L. Nail is an Adjunct Professor of Industrial and Physical Pharmacy at Purdue University and recently retired from the position of Senior Research Scientist at Baxter Biopharma Solutions, Bloomington, IN. His undergraduate training is in chemical engineering at Purdue University, and his Ph.D. is in pharmaceutics, also from Purdue. From 1975-1991 he worked for The Upjohn Company, Kalamazoo, MI in various capacities, all related to development and manufacture of parenteral products, with a special interest in the science and technology of freeze-drying. In 1991, he became Associate Professor in the School of Pharmacy at Purdue, and was promoted to Professor in 1999. His research interests at Purdue focused on the physical chemistry of freezing and freeze-drying, characterization of frozen systems and freeze-dried solids, stability of proteins as freeze-dried products, pharmaceutical thermal analysis, and pharmaceutical applications of supercritical fluid technology. His teaching responsibilities have included undergraduate pharmacy courses in parenteral pharmaceutical products and graduate courses in pharmaceutical processing. From 2002 until 2006, he was Research Fellow in the Pharmaceutical Sciences R&D organization at Eli Lilly & Co., Indianapolis, IN. He has served on the USP Committee of Experts in Parenteral Products from 1995 until 2010, and was the Chairman of this committee from 2005-2010. He is a Fellow of the American Association of Pharmaceutical Scientists. In 2007, he received the AAPS Research Achievement Award in Pharmaceutical Technology. He was recognized by the Purdue University School of Pharmacy as a Distinguished Alumnus in 2013. |
YouTube Training Videos
-
Interview Extra: Importance of Lyophilization for Cancer Therapy
-
Lyophilization/Freeze Drying of Mannitol vs Pure Water (time-lapse of 20 hour process)
-
Prof. Mike Pikal Lecture "Modeling in Freeze Drying: Past, Current State and Future Perspectives"
Interactive online tools for modeling and analysis of lyophilization/freeze-drying:
1. Interactive Lyocalculator
Lyocalculator models product temperature and sublimation rate during primary drying for specified chamber pressure, shelf temperature and container/product properties.
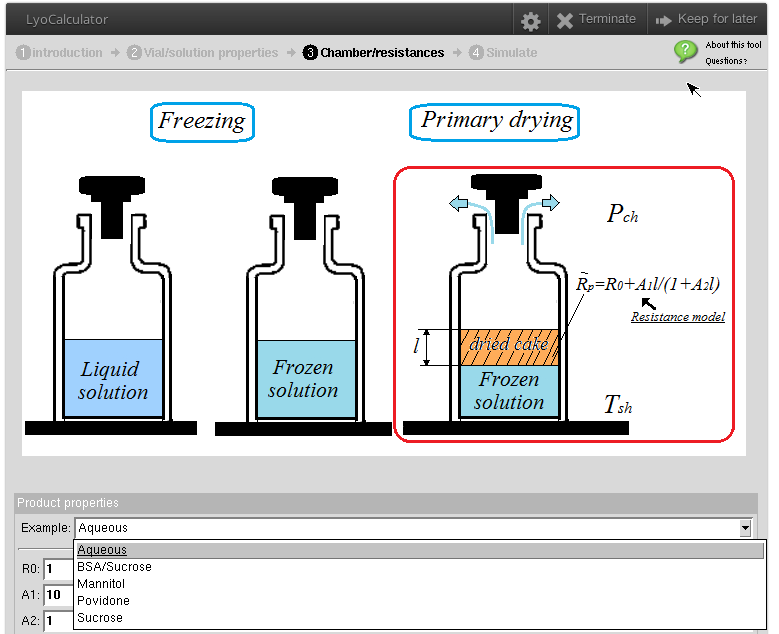
Excel-based lyocalculator (Courtesy Dr. Serguei Tchessalov/Pfizer):
Lyocycle_design_and_transfer_template_Breckenridge_workshop--Pfizer_Original.xls (2 MB)
Abridged version for training by Dr. Tong Zhu:
170727-Lyo-Calculator_Training-Added_Macro_Solver.xls (66 KB)
2. Pressure Variation Calculator
Lyo Chamber Pressure Variation Calculator
simulate variation of pressure within product chamber for specified chamber pressure, shelf size and drying rate rate.
Lyophilized Injectable Drug and Biological Product Database:
Comprehensive and searchable database containing all FDA-approved lyophilized injectable drug and biological products.



