You are here: Courses › Introduction to Lyophilization › Overview
Courses
Introduction to Lyophilization
This 8-module course will introduce participants to Lyophilization. Course also includes a lab exercise.

Online Lyophilization 101 Short Course
This 8-module course will introduce participants to Lyophilization. This course also includes a lab exercise. A self-assessment is available after each module.
Module 1: Introduction to Pharmaceutical Lyophilization, Presented by Dr. Elizabeth Topp, Purdue University
In this video, students will learn what lyophilization is and its importance for the pharmaceutical industry. The major parts of a lyophilizer are named and a scientific basis for lyophilization is provided, as well as the lyophilization cycle phases.
Module 2: Overview of the Lyophilization Process, Presented by Dr. Arnab Ganguly, IMA Life
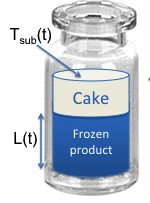
In this video, students will be introduced to freezing, primary drying and secondary drying and receive an overview of unit operations in production lyophilization.
Module 3: Production Lyophilizers, Presented by Dr. Alina Alexeenko, Purdue University
In this video, students will learn about the components of production lyophilizers including the chamber, tunnel, condenser, vacuum pump and refrigeration system. There is an introduction to sensors used to monitor temperature and pressure and current best practices.
Module 4: Glass Transition Temperature (Tg, Tg1), Presented by Dr. Akhilesh Bhambani, Merck and Co.
In this video, students will hear definitions of Tg and Tg’ and discover how Tg is measured using differential scanning calorimetry (DSC). Other topics include measurement of collapse temperature, estimation of Tg of mixtures, use of Tg and Tg’ in lyophilization cycle design and Tg and product stability.
Module 5: Freezing, Presented by Dr. Greg Sacha, Baxter
In this video, the thermodynamics of freezing and DSC thermograms, freeze concentration, eutectic mixtures, “skin” formation, freeze concentration and stability, controlled nucleation are discussed.
Module 6: Primary and Secondary Drying, Presented by Dr. Ehab Moussa, AbbVie
In this video, students are introduced to heat transfer (conduction, convection, radiation), mass transfer, vial heat transfer coefficient (Kv), measuring Kv, cake resistance (Rp), measuring Rp and spatial variation in drying of vials.
Module 7: Graphical Design Space, Presented by Dr. Steve Nail, Baxter
In this video, students will learn what sublimation rate vs. chamber pressure is and about the equipment capability curve, choked flow and choke point, shelf temperature and product temperature isotherms, Tg’ and collapse line, TDLAS and safe operating zone for lyophilization cycles.
Module 8: Quality Attributes of Lyophilized Products, Presented by Dr. Akhilesh Bhambani, Merck and Co.
In this video, students will learn about cake and vial appearance (collapse, partial collapse, shrinkage, cracking, fogging, lifting), rejectable vs. acceptable cosmetic defects, activity, sterility, reconstitution time, moisture content and stability and automated visual inspection.
VIRTUAL LAB EXERCISE IN PHARMACEUTICAL LYOPHILIZATION, Presented by Dr. Alina Alexeenko and Andrew Strongrich, Purdue University
In this video, students will learn learn how to develop and run a typical lyophilization cycle. This video was recorded in the LyoHUB Lyophilization Demonstration Facility at Purdue University.
To take the self-paced course, click on the "Start Course" button on the right side of this page.
This course created through the support of NIIMBL and LyoHUB