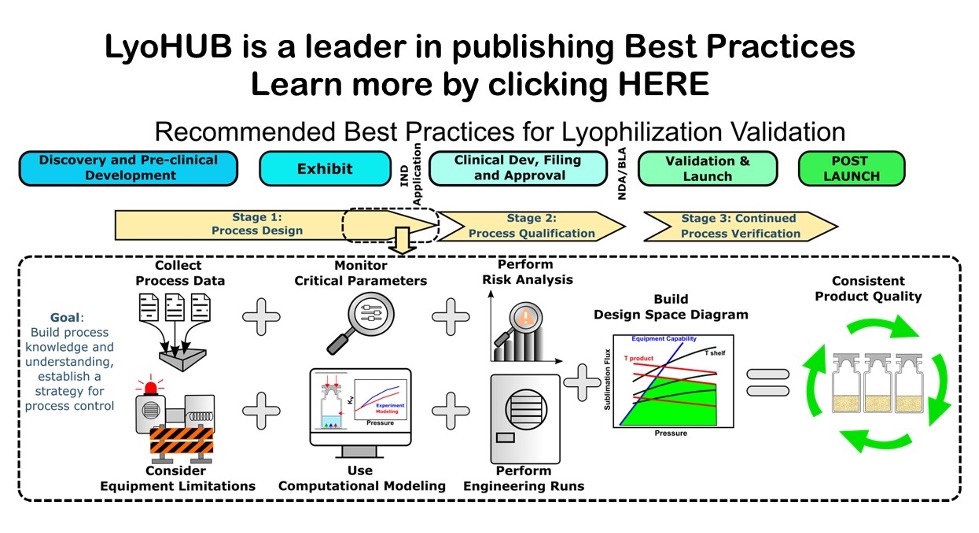
Recommended Best Practices for Lyophilization Validation-2021 Part I: Process Design and Modeling an
In 2021, LyoHUB’s Recommended Best Practices for Lyophilization Validation, Parts 1 & 2 outlines best practices for lyophilization process validation and have been published in AAPS PharmSciTech. These papers have been made available in open source by LyoHUB and can be downloaded at no cost. Paper 1 has been downloaded over 4,976 times and is available at https://link.springer.com/article/10.1208/s12249-021-02086-8 Paper 2 has been downloaded over 3,309 times and is available at https://link.springer.com/content/pdf/10.1208/s12249-021-02107-6.pdf
The papers’ contents are listed below:
The best practices paper Part 1: Process Design and Modeling describes lyophilization process validation and consists of two parts. Part one focuses on the process design and is described in the current paper, while part two is devoted to process qualification and continued process verification. The intent of these papers is to provide readers with recent updates on lyophilization validation in the light of community-based combined opinion on the process and reflect the industrial prospective.
In this paper, the design space approach for process design is described in detail, and examples from practice are provided. The approach shows the relationship between the process inputs. It is based on first principles and gives a thorough scientific understanding of process and product. The lyophilization process modeling and scale-up are also presented showing the impact of facility, equipment, and vial heat transfer coefficient. The case studies demonstrating the effect of batch sizes, fill volume and dose strength to show the importance of modeling as well as the effect of controlled nucleation on product resistance are discussed.
The best practices paper Part 2: Process Qualification and Continued Process Verification is devoted to process qualification and continued process verification. The goal of the study is to show the cutting edge of lyophilization validation based on the integrated community-based opinion and the industrial perspective. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process parameters selection strategies, and batch size overage to compensate for losses during production. It also includes sampling strategies to demonstrate batch uniformity as well as the use of statistical models to ensure adequate sampling.
Based on the LyoHUB member organizations survey, the best practices in determining the number of PPQ runs are developed including the bracketing approach with minimum and maximum loads. Standard practice around CQA and CPP selection is outlined and shows the advantages of using control charts and run charts for process trending and quality control.
The case studies demonstrating the validation strategy for monoclonal body and the impact of the loading process on the lyophilization cycle and product quality as well as the special case of lyophilization for dual-chamber cartridge system are chosen to illustrate the process validation. The standard practices in the validation of the lyophilization process, special lyophilization processes, and their impact on the validation strategy are discussed. Additionally, special cases of the lyophilization in alternate primary packaging systems such as dual-chamber vials, syringes, and cartridges are discussed, and recommended best practices for alternate lyophilization container-closure systems are suggested.