Resources
In the pharmaceutical industry, time and productivity are lost due to the lack of scientifically sound and industry-standard approaches to issues such as batch acceptance, equipment qualification, process validation, cleaning validation, and leak-rate acceptance. In the absence of such “best practices,” companies often take overly conservative approaches that lead to rejection of product and lengthy delays in the start-up of new equipment and facilities.
Best Practice Papers Published to date (Details and links for each paper located below)
Practical Advice on Scientific Design of Freeze Drying Process: 2023 Update
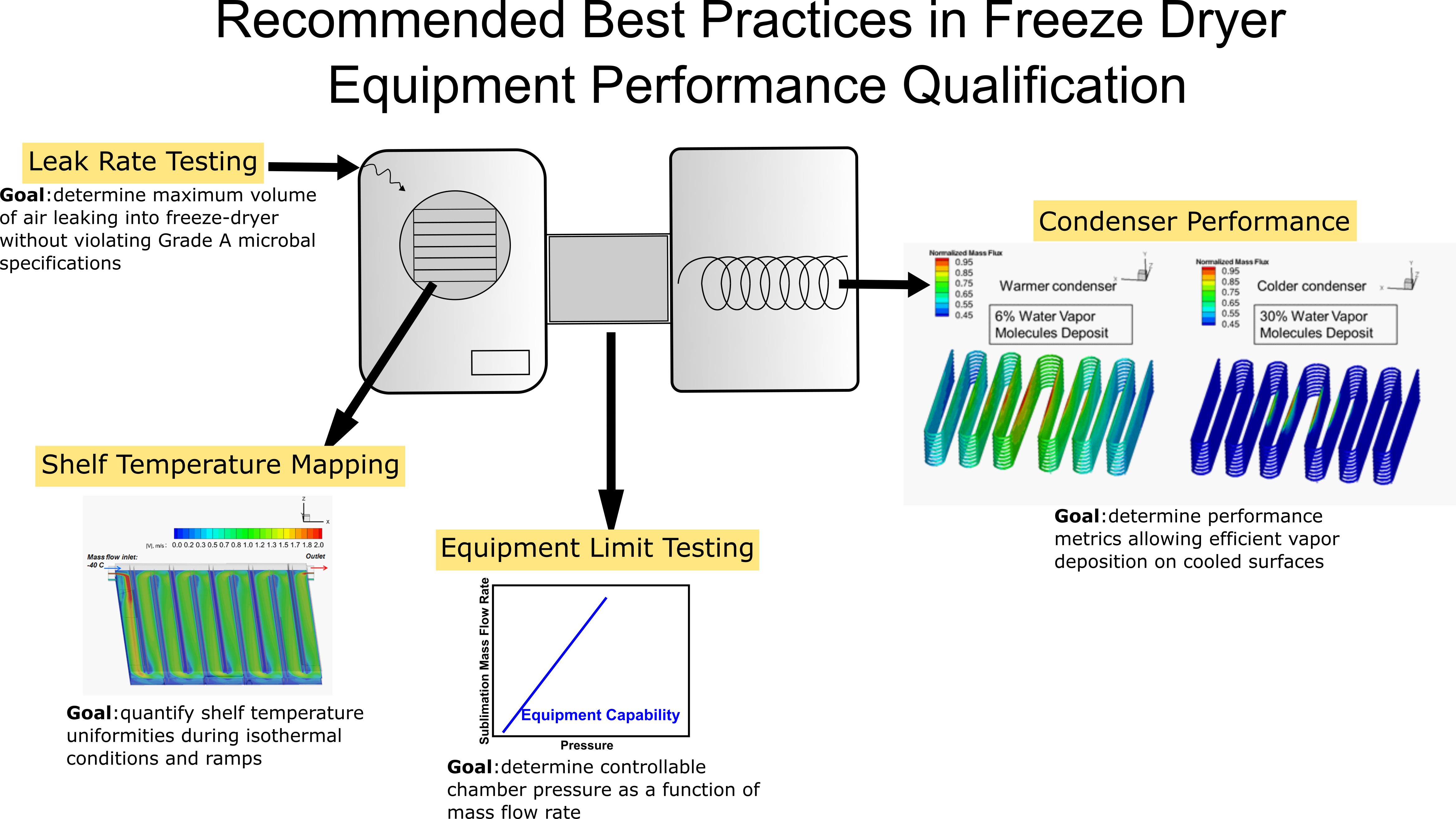
Recommended Best Practices in Freeze Dryer Equipment Performance Qualification: 2022
Best Practices and Guidelines (2022) for Scale-Up and Tech Transfer in Freeze-Drying Based on Case Studies. Part 1: Challenges during Scale Up and Transfer
Recommended Best Practices for Lyophilization Validation—2021 Part I: Process Design and Modeling
Practical Advice on Scientific Design of Freeze-Drying Process: 2023 Update
The purpose of this paper is to re-visit the design of three steps in the freeze-drying process, namely freezing, primary drying, and secondary drying steps. Specifically, up-to-date recommendations for selecting freeze-drying conditions are provided based on the physical–chemical properties of formulations and engineering considerations. The authors revisited advice from a seminal paper by Tang and Pikal (Pharm Res. 21(2):191-200, 2004) on selecting freeze-drying process conditions and found that the majority of recommendations are still applicable today. There have been a number of advancements, including methods to promote ice nucleation and computer modeling for all steps of freeze-drying process. The authors created a database for primary drying and provided examples of complete freeze-drying cycles design. The paper may supplement the knowledge of scientists and formulators and serve as a user-friendly tool for quickly estimating the design space.
Recommended Best Practices in Freeze Dryer Equipment Performance Qualification: 2022
Best practices for performing freeze dryer equipment qualification are recommended, focusing on identifying methods to quantify shelf thermal uniformity (also known as “shelf surface uniformity”), equipment capability, and performance metrics of the freeze dryer essential to the pharmaceutical Quality by Design paradigm. Specific guidelines for performing shelf temperature mapping, freeze dryer equipment limit testing (the capability curve), and condenser performance metrics have been provided. Concerning shelf temperature mapping and equipment capability measurements, the importance of paying attention to the test setup and the use of appropriate testing tools are stressed. In all the guidelines provided, much attention has been paid to identifying the balance between obtaining useful process knowledge, logistical challenges associated with testing in the production environment vs that at laboratory scale, and the frequency of the testing necessary to obtain such useful information. Furthermore, merits and demerits of thermal conditions maintained on the cooled surfaces of the freeze dryer condenser have been discussed identifying the specific influence of the condenser surface temperature on the process conditions using experimental data to support the guidelines. Finally, guidelines for systematic leak rate testing criteria for a freeze dryer are presented. These specific procedural recommendations are based on calculations, measurements, and experience to provide useful process and equipment knowledge.
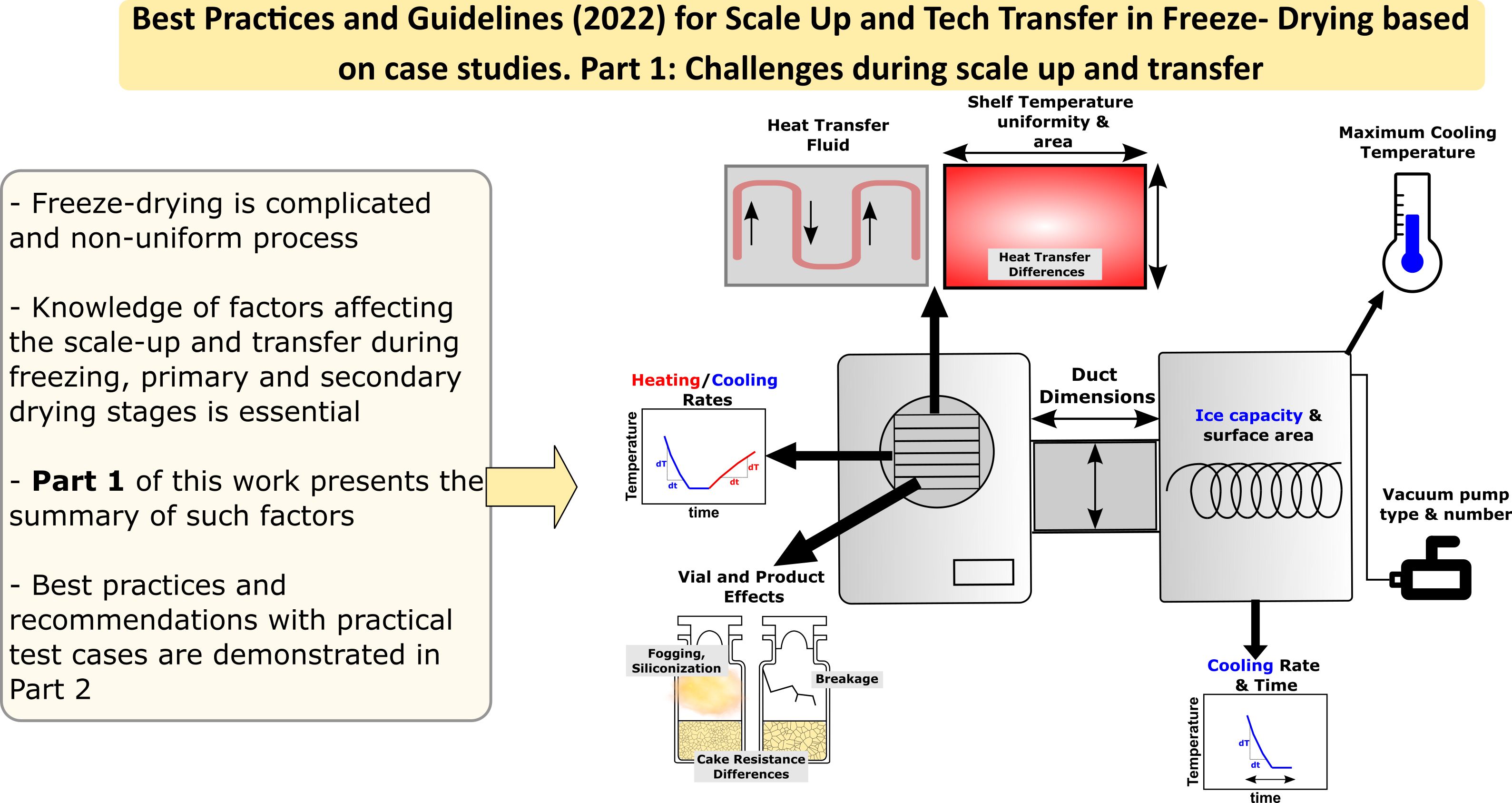
Best Practices and Guidelines (2022) for Scale Up and Tech Transfer in Freeze-Drying based on case studies. Part 1: Challenges during scale up and transfer
The freeze-drying process scale up and transfer remain a complicated and non-uniform practice. This paper summarizes inefficient and good practices in these papers and provided some practical advice. It was demonstrated that using the same process set points/times in laboratory and commercial scale dryers may lead to loss of product quality (collapse or vial breakage). The emerging modeling approach demonstrated practical advantages demanding. However, the upfront generation of some input parameters (vial heat transfer coefficient, minimum controllable pressure, and maximum sublimation rate) is essential for the model(s) utilization. While the primary drying step can be transferred with a high degree of confidence (e.g., using modeling), and secondary drying is usually fairly straightforward, predicting potential changes in product behavior during freezing remains challenging.
This paper was completed in collaboration with BioPhorum.
Recommended Best Practices for Lyophilization Validation-2021 Part I: Process Design and Modeling and Part II; Process Qualification and Continued Process Verification now available for free download here on LyoHUB website
In 2021, LyoHUB’s Recommended Best Practices for Lyophilization Validation, Parts 1 & 2 outlines best practices for lyophilization process validation and have been published in AAPS PharmSciTech. These papers have been made available in open source by LyoHUB and can be downloaded at no cost. Paper 1 has been downloaded over 4,976 times and is available at https://link.springer.com/article/10.1208/s12249-021-02086-8 Paper 2 has been downloaded over 3,309 times and is available at https://link.springer.com/content/pdf/10.1208/s12249-021-02107-6.pdf
The best practices paper Part 1: Process Design and Modeling describes lyophilization process validation and consists of two parts. Part one focuses on the process design and is described in the current paper, while part two is devoted to process qualification and continued process verification. The intent of these papers is to provide readers with recent updates on lyophilization validation in the light of community-based combined opinion on the process and reflect the industrial prospective.
In this paper, the design space approach for process design is described in detail, and examples from practice are provided. The approach shows the relationship between the process inputs. It is based on first principles and gives a thorough scientific understanding of process and product. The lyophilization process modeling and scale-up are also presented showing the impact of facility, equipment, and vial heat transfer coefficient. The case studies demonstrating the effect of batch sizes, fill volume and dose strength to show the importance of modeling as well as the effect of controlled nucleation on product resistance are discussed.

The best practices paper Part 2: Process Qualification and Continued Process Verification is devoted to process qualification and continued process verification. The goal of the study is to show the cutting edge of lyophilization validation based on the integrated community-based opinion and the industrial perspective. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process parameters selection strategies, and batch size overage to compensate for losses during production. It also includes sampling strategies to demonstrate batch uniformity as well as the use of statistical models to ensure adequate sampling.
Based on the LyoHUB member organizations survey, the best practices in determining the number of PPQ runs are developed including the bracketing approach with minimum and maximum loads. Standard practice around CQA and CPP selection is outlined and shows the advantages of using control charts and run charts for process trending and quality control.
The case studies demonstrating the validation strategy for monoclonal body and the impact of the loading process on the lyophilization cycle and product quality as well as the special case of lyophilization for dual-chamber cartridge system are chosen to illustrate the process validation. The standard practices in the validation of the lyophilization process, special lyophilization processes, and their impact on the validation strategy are discussed. Additionally, special cases of the lyophilization in alternate primary packaging systems such as dual-chamber vials, syringes, and cartridges are discussed, and recommended best practices for alternate lyophilization container-closure systems are suggested.

Best Practices in Instrumentation for Process Monitoring in Pharmaceutical Freeze-Drying - 2016
Timeline:
* April 2014: LyoHub initiated development of Best Practices in Instrumentation for Process Monitoring in Pharmaceutical Freeze Drying. Working group was led by Dr. Steve Nail (retired from Baxter BioPharma) and includes lyophilization experts from Pfizer, Allergan, IMA Life, Physical Sciences, University of Connecticut, Purdue University, IMA Life and Millrock Tech.
* April 2015: Best Practices Presentation at 2015 ISLFD Meeting in Chicago, April 9, 2015. Community input requested.
* July 2016: The draft of recommended best practices presented at 2016 CPPR Freeze Drying of Pharmaceuticals & Biologicals Conference in Breckenridge, CO.
* September 2016: Best Practices Presentation at 2016 Aseptic Processing Symposium in Buffalo, NY, September 14, 2016.
* November 2016: Best Practices Brochure for "Process Instrumentation in Freeze Drying" introduced. Click here to download a copy of the brochure to keep in your lab.
* March 2017: Best Practices Paper provided by LyoHUB in open source. To access the complete best practices paper, visit http://link.springer.com/article/10.1208/s12249-017-0733-1
* 2021: First Standard for Lyophilization issued through ASTM, ASTM E3250 - 21 Standard Practice for Product Temperature and Equipment Pressure Instrumentation in Pharmaceutical Freeze Drying

Summary of recommendations - 2016:
1. Recommendations for measuring product temperature in individual vials
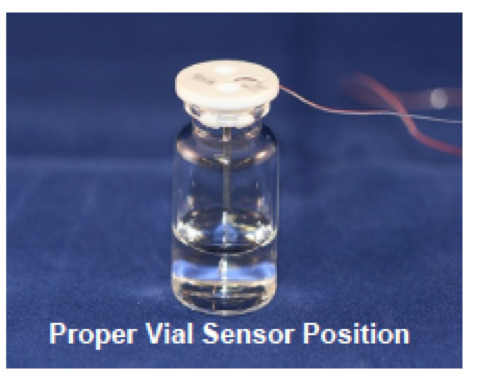
A) Thermocouples are preferred to other current methods because of the ability to measure temperature at a precise point. The most appropriate point to measure product temperature is in the center of the vial, with the tip of the thermocouple touching the bottom of the vial.
B) Use fine wire (e.g. 32 gauge) thermocouples because of the flexibility of the wire and the ability to locate the tip of the thermocouple in a precise location.
C) Use a guide tube to hold the thermocouple in place within a monitored vial. The open area of this device should be very close to that of a partially stoppered vial.
D) Be aware of the sources of uncertainty associated with product temperature measurement in a manufacturing setting, and don’t over-interpret such data.
E) Be aware of the bias in freezing and freeze-drying behavior caused by any temperature measuring device. Recognize that monitored vials may freeze dry significantly faster than the rest of the batch.
F) The same best practices that apply at the laboratory scale should also apply in a manufacturing environment.
2. Recommendations for measuring chamber pressure in freeze-drying
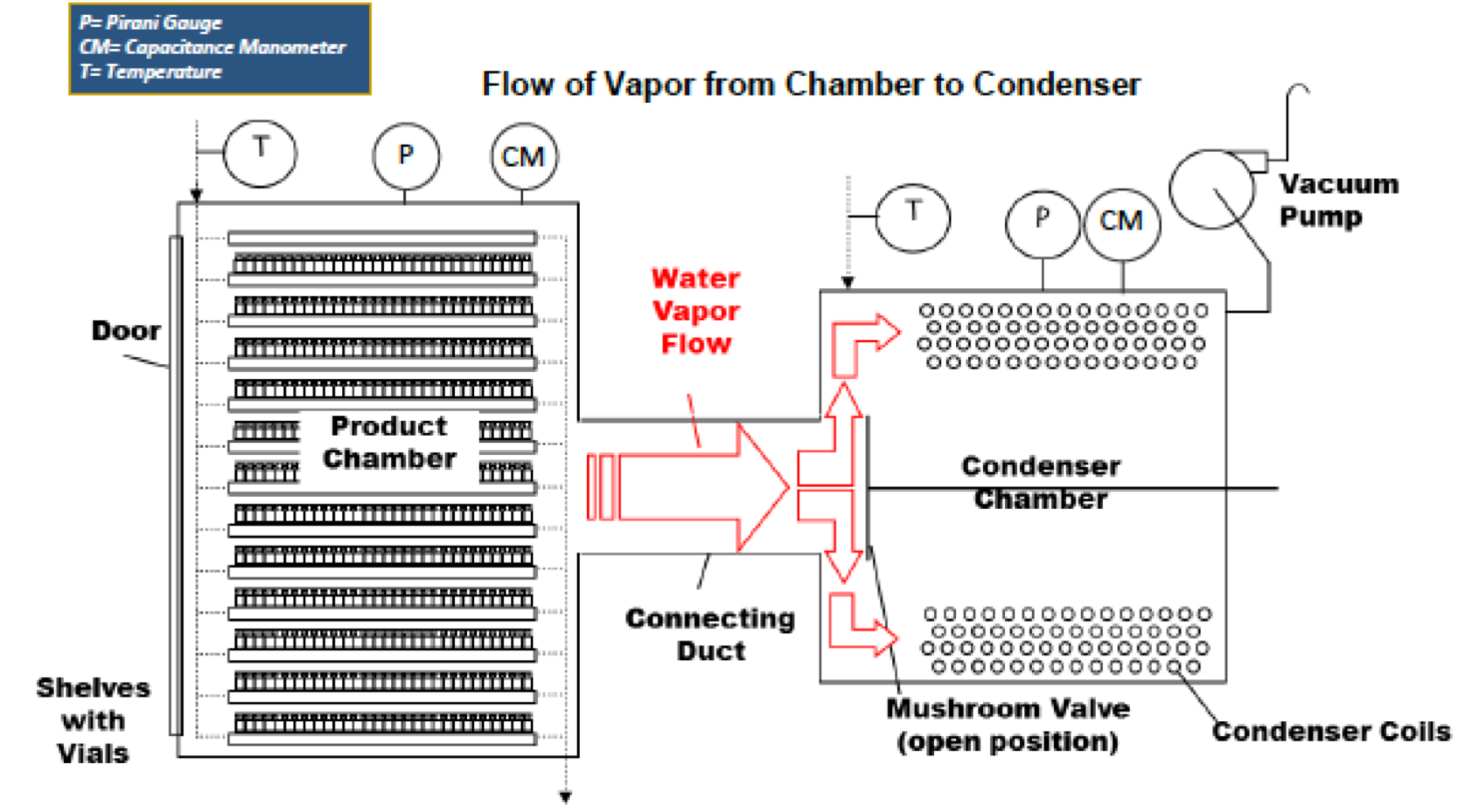
A) The capacitance manometer is the instrument of choice for pressure measurement and control in a pharmaceutical freeze dryer. A temperature-controlled sensing head is highly recommended.
B) Best practice would include both a capacitance manometer and a Pirani gauge on both the chamber and the condenser.
C) Use of comparative pressure measurement is highly recommended as a process monitoring tool to determine the end point of both primary and secondary drying.
D) Keep in mind that both repeated exposure to atmospheric pressure and repeated steam sterilization tend to shorten the interval between calibrations of a capacitance manometer. Historical records are useful in establishing the more appropriate time interval between calibrations. In situ calibration is not considered best practice.
Please contact us with your comments and suggestions on best practices in lyophilization/freeze-drying.
Comments
There are no comments on this entry.



