You are here:
SafeRx Dashboard
- Details
- Created on Tuesday, 05 December 2017 16:57
- Last Updated on Friday, 17 September 2021 10:18
- Published on Monday, 04 December 2017 05:57
- Hits: 0
| The SafeRx project is a collaboration between the Center for Medication Safety Advancement (CMSA) at the College of Pharmacy and the Rosen Center for Advanced Computing at Purdue University. See SafeRx Database Enables Large-scale of Prescription Medication Safety Only authorized users can access the SafeRx Database. | |||

Data Explorer for FDA FAERS DataYou can enter search criteria for drugs, reactions, outcomes, demographics and more! Dataviews generate in seconds, and you can use the column filters to refine your search as you investigate ADEs for your selected drugs.Click on The FDA FAERS Data Explorer to begin ... USE FIREFOX OR CHROME (no Internet Explorer please!) Tips for using your Data Explorer Make sure your browser allows pop-up windows. Review the FDA FAERS Data Dictionary  Click any magnifying glass to search the complete FAERS list of choices for your criteria. There are more than 200,000 choices for Drugs - the search list helps you review all possibilities. Copy and paste from the search list for your criteria. Click any magnifying glass to search the complete FAERS list of choices for your criteria. There are more than 200,000 choices for Drugs - the search list helps you review all possibilities. Copy and paste from the search list for your criteria. Hover over any checkbox for details on how it affects your search. Hover over any checkbox for details on how it affects your search. |
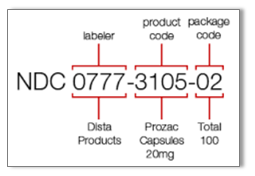
Dataviews for FDA NDC Data
Package Data: shows the package description and NDC package code. The product ID links to the NDC product. Product Data: shows the complete NDC manufacturer product information. |
||
| SafeRx was used by the Evidence-based Practice Course in Purdue University's School of Nursing to compare data from Twitter social media tweets to adverse drug events data reported to the FDA | |||
Database features available for authorized SafeRx administrators only
LOAD NEW FDA DATA Update SafeRx Update SafeRxFAERS and NDC |
SAFERX ACCESS GROUP Invite Members Invite Members |
SANDBOX  AU Meds AU Meds |
FAERS Report Counts by Month 2012-2016  |
Top 20 Drugs & Reactions by County  |
Medication Safety and Adverse Events: Dataviews are used for research investigations into medication safety -- to find answers and to discover links for adverse drug events. SafeRx presents data from FDA Adverse Event Reporting System (FAERS) and from FDA National Drug Code Directory. New sources of adverse drug event data will be added to SafeRx on an ongoing basis. Users can search, filter, link, explore and download adverse drug events data from the SafeRx Database. FAERS Data Elements: Definition, Interpretation and Assessment (SaferRx administrators only)
The FDA FAERS "Raw Data" Dataviews (SafeRx administrators only)All Dataviews have Primary and Case IDs that link to Case Summary Reports. Drugs in the Case Summary Reports are primary suspect or secondary suspect. These are very long-running dataviews. Use them only when you want to view all FAERS data WITHOUT a search filter. Otherwise, you should use the FAERS Data Explorer.
|
FAERS Analytics Long-Running Dataviews (SafeRx administrators only)
| Demographic Statistics events by gender, age, weight -
|
Reporting Fequencies reports by source, geography,manufacturer. Report totals link to drug list.
|
Drug and Reaction Detail correspondence of reactions and drugs
|