You are here: resources › Databases › NIPTE-FDA Excipients Knowledge Base › About
NIPTE-FDA Excipients Knowledge Base
Category
Published on
Abstract
pharmaHUB now offers its research community a publicly available Excipients Knowledge Base that contains material properties and usage data that are useful for formulation development and the evaluation of formulations by regulators.
As our Excipients Knowledge Base grows, we plan to add knowledge management tools that can be used to aid formulation development and excipient evaluation and comparison.
Click the black View Link button above to begin your exploration.
The NIPTE-FDA Excipients Knowledge BaseA collaborative effort betweenFood and Drug Administration (FDA) National Institute for Pharmaceutical Technology and Education (NIPTE) Purdue University Rosen Center for Advanced Computing The Pharmaceutical Excipient Knowledge Base is an online resource that offers the pharmaHUB community a searchable repository for the newest excipient property measurement data
|
Conferences
NIPTE RESEARCH CONFERENCE
Understanding Excipient Performance – Key to Successful QbD Formulation Design
June 13-14, 2012
FDA Conference Facilities White Oak, Silver Spring, MD
- Conference Brochure
- Conference Agenda
- Ann Christine Catlin The NIPTE-FDA Excpient Database Hosted at pharmaHUB (powerpoint show)
AIChE, October, 2011
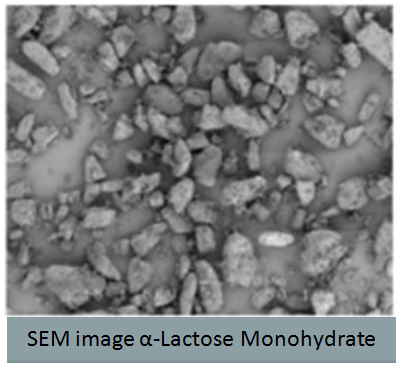
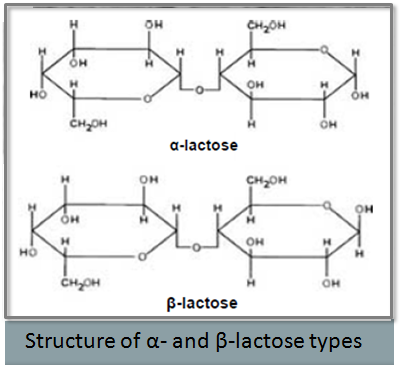
- Investigation of Mechanical Property Variability in Lactose Products presented by Kristine Alston
FDA, August, 2011
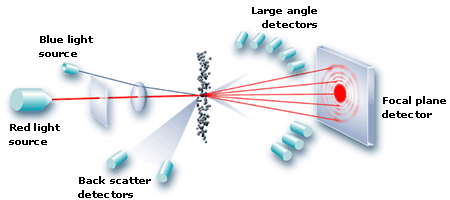
- Plan for Approach to the Understanding and Predicting Excipient Properties and Functionality presented by Steven Hoag
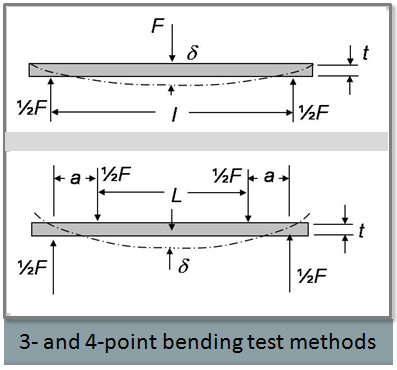
- Excipient Mechanical Properties and Measurements presented by Carl Wassgren
- The NIPTE-FDA Excipients Knowledge Base Hosted at pharmaHUB presented by Ann Christine Catlin
Other Materials
Cite this work
Researchers should cite this work as follows:
Location
http://pharmahub.org/resources/448