You are here:
The NIPTE-FDA Excipient Risk Assessment Database
- Details
- Created on Wednesday, 23 August 2017 11:25
- Last Updated on Monday, 11 June 2018 11:39
- Published on Tuesday, 22 August 2017 11:25
- Hits: 0

Excipients play a critical role in the manufacturability and clinical performance of dosage forms. To evaluate the risks associated with excipient selection, characterization, and use, we developed a risk analysis decision support tool. The tool couples risk assessment and risk narratives with catalogs of excipients, dosage form types, functionalities, and manufacturing methods. Our tool can be used by industry during development to provide sound integrated risk analysis that addresses questions and concerns about stability, drug delivery performance, direct and indirect effects related to impurities and toxicity, and other issues. It can also be used by the FDA for integrated NDA reviews and efficient knowledge transfer to CGMP inspectors on risk factors to consider during their approval processes for product quality and safety.
Principal Investigators: Stephen W. Hoag (University of Maryland) and Vadim Gurvich (NIPTE)
Data Platform R&D: Ann Christine Catlin, Chandima Hewa Nadungodage, Andres Bejarano (Purdue University)
Research Associate: Fang Wang (University of Maryland)
Funded by NIPTE and the FDA.
Decision Support for Excipient Risk Assessment
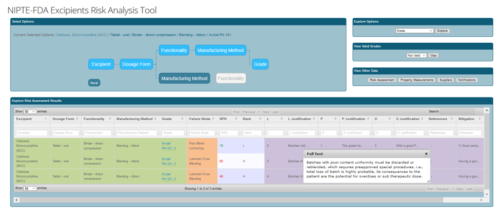 |
Our database manages the complexity of relationships between excipients and their valid dosage forms, functionalities, and manufacturing methods. For each valid relationship, the database associates risk factors and RPN analyses, along with references and mitigation strategies. Our decision support tool guides users through selection of valid options and produces a full risk analysis report for the chosen excipient, dosage form, functionality, manufacturing method, and grade. Click here to begin. |
With the excipient risk assessment tool, users can also explore
* Grades that remain valid as users select excipient, dosage form, functionality and manufacturing method
* Suppliers for each grade
* Rules for the valid functionality, dosage forms and manufacturing methods for each choice of excipient
* Grade properties and measurements
Documents for the risk assessment database
| User Documentation User Guide |
Instructions for using the Excipient Risk Assessment Tool |
| Master Lists Excipients, Dosage Forms, Functionality, Manufacturing Methods, Grades |
Spreadsheets that list the excipients, grades, functionality categories, dosage forms and manufacturing methods that are available in the risk assessment database. In the Grades Master List, the Excipient and Supplier for each grade are also listed. |
| Knowledge-based Rules Grade-Functionality, Grade-Dosage Form, Grade-Manufacturing Method, Functionality-Dosage Form, Functionality-Manufacturing Method, Manufacturing Method-Dosage Form |
Spreadsheets with rules that identify all valid relationships between grades, functionality, dosage form, and manufacturing method. The relationships determine the selections that can be made in the decision support process and the combinations that require risk assessment data. |