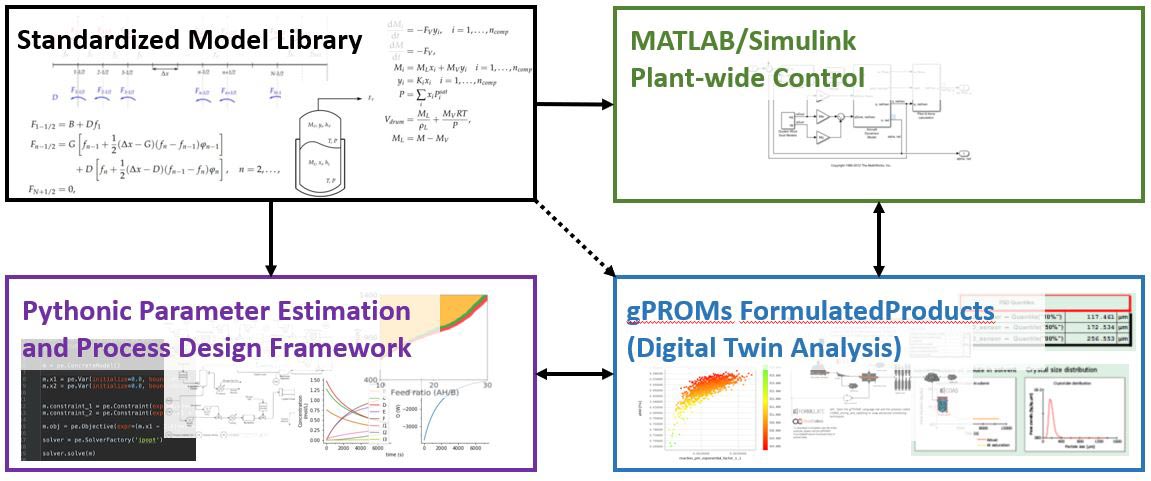
In recent years, the integration of continuous manufacturing in the pharmaceutical industry has received great attention regarding the reported benefits over traditional batch-wise operation, such as the reduction of batch-to batch quality variability, and a reduction in equipment sizes, among others. However, quantitative tools and a standardized framework to analyze and ultimately decide which mode of manufacturing or combination of manufacturing modes best suits a given pharmaceutical process either do not exist or are tailored to specific applications. Also, decisions are typically made largely on a heuristic basis and may overlook complex equipment relationships that result in a more productive or less wasteful processing scheme. The goal of the platform is to provide a standardized model library as well as a suite of quantitative analysis tools and techniques that will allow the user to perform end-to-end analysis of a pharmaceutical process, promoting efficient manufacturing practices through optimal process design and control.
The framework has been divided into three major components: (1) The parameter estimation and optimal design framework, (2) The in-depth process simulation platform and (3) The process control platform. Each component of the framework requires differing strengths with respect to modeling and numerical solution capabilities. Therefore, each component has been constructed in a software platform whose strengths align closely with the given task.
The first component is PharmaPy, a Pythonic framework for preliminary process simulation and parameter estimation in the early design and experimentation stages. PharmaPy is equipped with state-of-the-art integration routines for the solution of dynamic batch and continuous unit operation models, and in-house optimization routines for parameter estimation and the statistical analysis of parameter estimates. The parameter estimation results may be readily imported into the optimal design framework. Here, Pyomo is utilized to analyze optimal single unit operation design as well as generating and solving design superstructure problems. Pyomo has interfaces to powerful, state-of-the-art numerical solvers which are required when analyzing nonlinear dynamic models and large MILPs/MINLPs resulting from superstructure formulations.
The second component utilizes gPROMs FormulatedProducts. Here, results from the superstructure analysis will be tested for feasibility and practicality using Digital Twins. The existing graphical user interface and simulation flexibility that is offered in gPROMs FormulatedProducts is perfectly suited for confirming the reliability of output from the Python-based framework in component 1. Also, capability of simulating process disturbances and monitoring CQAs can provide crucial information for quantifying or confirming a desired design space. Finally, the control component is built within MATLAB Simulink. Here, complex plant-wide control schemes may be developed and analyzed on feasible process designs from components (1) and/or (2). Coinciding closely with gPROMs FormulatedProducts, introducing disturbances and analyzing plant-wide performance is vital to operate a continuous or hybrid pharmaceutical manufacturing operation to the maximum potential.
For the components to work in concert, a standardized model library will be provided over each platform. The physics and implementation of these models in Python/Pyomo and MATLAB that align relatively well with gPROMs implementations is shown as supplemental material through this website. Also, teaching material and example flowsheets are provided to help the user get started and understand the various capabilities of the platform.